- New Service Alert: Sequence now offers comprehensive Smoke Studies services!
Improving the quality of human life is the heart of everything we do.
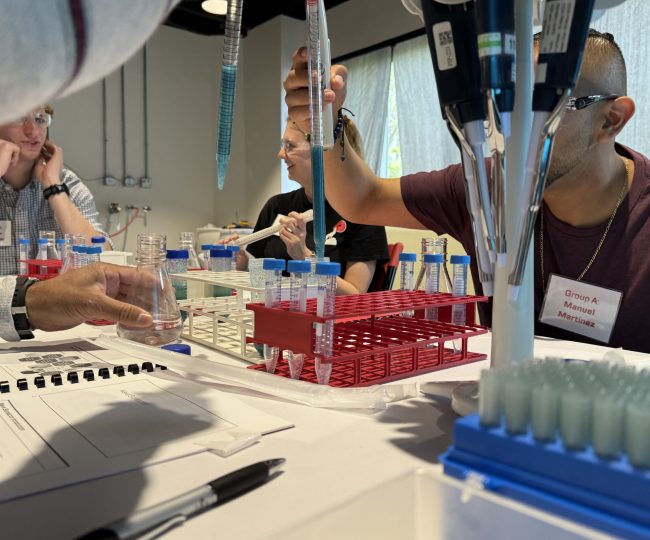
Sequence offers hands-on training for pharmaceutical manufacturing operations. Our fully functional mock pharmaceutical facility allows us to train teams in five key areas: Facilities, Utilities, Equipment, Computerized Systems and QC Lab.
Courses are offered in both standard curriculum courses and customized course options to fit a variety of needs. A key benefit of customized training at Sequence is our ability to incorporate your internal organization’s SOPs and other procedures to develop a personalized program for your team.


Since 2002, Sequence has offered consulting services for some of the top pharmaceutical manufacturers in the world. Our in-depth understanding of equipment and processes equips our team with the ability to teach the concepts as well as develop customized curriculums that are easy to understand and immediately impactful in terms of product quality, patient safety and driving results to the bottom line.
Our training team at Sequence has decades of on-site experience supporting our biopharmaceutical clients. This insight brings a critical level of effectiveness and perspective to our training programs.

Our training program is focused on making sure our employees develop an understanding of how to apply both regulation and guidance to the validation lifecycle, specifically focusing on design, commissioning, and qualification of new facilities.
The combination of hands-on training together with theory enhances understanding of highly technical processes that can’t be fully understood through textbooks or classroom training alone. Interacting directly with equipment and systems, allows operators to gain a better understanding of how each piece of machinery functions within the larger manufacturing process. This allows them to troubleshoot, make adjustments, and anticipate issues before they arise.
Hands-on training allows operators to better understand the regulatory and compliance requirements of their work. The ability to apply this knowledge in a practical setting ensures higher compliance rates and minimizes the risk of costly regulatory violations.
Operators trained hands-on are better at managing the intricacies of each production batch, which leads to more consistent product quality and fewer batch failures or rejections.
The pharmaceutical industry is fast-paced and problems can arise unexpectedly. Operators who have practical training can more quickly diagnose and resolve these issues. They are also more likely to feel confident in making critical decisions under pressure, which is vital in ensuring the smooth operation of production and maintaining product quality.
Hands-on training ensures that operators are not only familiar with SOPs but can apply them consistently and accurately. Through hands-on training, operators gain the practical experience needed for regular calibration and maintenance of equipment, ensuring that machinery runs optimally and that any downtime is minimized.
Operators who are trained in a controlled, hands-on environment are more likely to identify potential safety hazards and take preventive measures. During hands-on training, operators can practice emergency response procedures which can help them remain calm and effective in critical situations.
New operators can quickly get up to speed with hands-on training. This is particularly important in the pharmaceutical industry, where the ability to operate and troubleshoot machinery promptly is key to maintaining production timelines.
This site uses cookies for analytical purposes. No user identifiable data is collected or shared. If you agree to our use of cookies, please accept and continue.
No Code App Development for Manufacturing Operations
Tulip creates user-friendly and functional apps that improve the productivity of operations, without writing any code. Users can start from scratch or customize an App Template to fit their needs.
Sequence partners with Tulip for implementation and validation of the Tulip platform as well as creation, configuration and validation of Tulip Applications that are hosted on the platform. Our application builds include Electronic Batch Records, Weigh and Dispense, Digital SOPs for training (with embedded videos) and more.