Smoke Studies
Professional smoke studies for pharmaceutical and biotech applications. GMP-ready smoke testing services designed to visualize airflow patterns, validate contamination control, and ensure compliance with FDA, EU GMP, and global regulatory requirements.
Complete Smoke Studies Expertise
Smoke studies are critical testing procedures that visualize airflow patterns in cleanrooms and controlled environments. Sequence brings more than 22 years of hands-on experience supporting life science manufacturers with complex facility and process challenges. As an engineering consulting firm, Sequence focuses on ensuring that facilities, utilities, equipment, and manufacturing processes are designed, qualified, and tested to meet end user and regulatory requirements throughout the full project lifecycle.
This deep process and CQV expertise allows our team to integrate smoke study insights into broader contamination control and operational readiness strategies, helping clients achieve compliant, efficient, and reliable operations from initial design through routine production.
Where We Apply Our Expertise
Laminar Airflow Testing
(BSC, Fume Hoods)
Cleanroom Validation
HVAC System Verification
Isolators
Facility Design & Optimization
Regulatory Compliant Execution
Precision Smoke Studies Airflow Visualization
Watch how our advanced smoke study techniques reveal critical airflow patterns in cleanroom environments
GMP-Ready Equipment & Personnel
Our certified technicians utilize state-of-the-art smoke generation equipment designed specifically for pharmaceutical and biotech cleanroom environments.
- Trained & certified cleanroom personnel
- Professional-grade smoke generation systems
- High-resolution video documentation
- Real-time analysis capabilities
- Comprehensive regulatory compliance
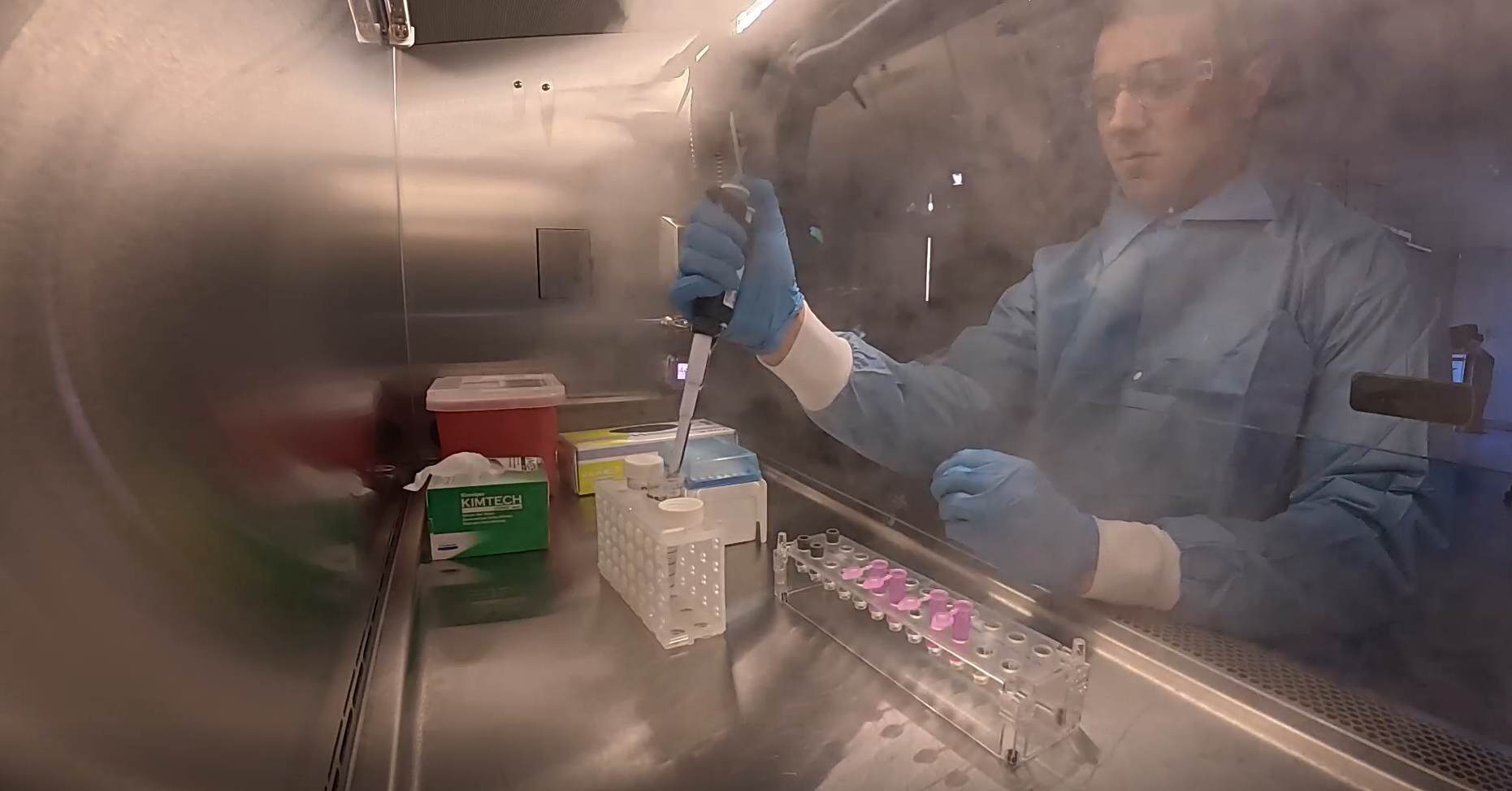
Turnkey Smoke Studies Solutions
From protocol development to final execution, we manage every aspect of your airflow visualization project.
Custom High-Resolution Video Production
Professional documentation with detailed visual analysis to support protocol execution and regulatory submissions.
Protocol Generation & Onsite Execution
Comprehensive protocol development tailored to your facility requirements, followed by expert onsite execution.
GMP Ready Equipment
State-of-the-art smoke generation equipment operated by certified professionals with extensive cleanroom experience.
Cost-Effective & Timely Execution
Streamlined processes designed for reduced downtime and efficient scheduling to minimize impact on operations.
Procedural Optimization
Leverage study results to optimize cleanroom procedures, material flows, and personnel movements for enhanced contamination control.
Complete Turnkey Solution
From initial consultation through final report delivery, we manage every aspect of your smoke study project.
Built on Compliance Excellence
Our smoke study services align with global regulatory requirements and industry best practices, ensuring your facility meets the highest standards.
International Society for Pharmaceutical Engineering standards
Guidance for Industry: Aseptic Processing
Annex 1 Compliance
Parenteral Drug Association Guidelines
Cleanroom & Controlled Environments
Current Good Manufacturing Practice
How We Work ▼
Click to view our 5-step process
Initial Consultation
We assess your facility requirements, regulatory needs, and project objectives to develop a customized approach.
Protocol Development
Our team creates comprehensive, site-specific protocols that align with your SOPs and regulatory requirements.
Onsite Execution
Certified technicians perform smoke studies using professional-grade equipment with minimal disruption to operations.
Analysis & Reporting
Detailed analysis with high-resolution video documentation and actionable recommendations for optimization.
Ongoing Support
Post-project support to implement recommendations and ensure continued compliance and performance.
Ready to Validate Your Cleanroom Airflow?
Partner with the experts who understand both the science and compliance requirements of airflow visualization.
Get Started Today →